
Resorcinarene‐Based o‐Biarylphosphines in Palladium‐Catalysed Suzuki–Miyaura Cross‐Coupling Reactions of Bulky Substrates - Elaieb - 2017 - European Journal of Inorganic Chemistry - Wiley Online Library

Convenient and General Palladium‐Catalyzed Carbonylative Sonogashira Coupling of Aryl Amines - Wu - 2011 - Angewandte Chemie International Edition - Wiley Online Library

Carbonylation of terminal alkynes catalysed by Pd complexes in combination with tri(2-furyl)phosphine and methanesulfonic acid - ScienceDirect

Resorcinarene‐Based o‐Biarylphosphines in Palladium‐Catalysed Suzuki–Miyaura Cross‐Coupling Reactions of Bulky Substrates - Elaieb - 2017 - European Journal of Inorganic Chemistry - Wiley Online Library
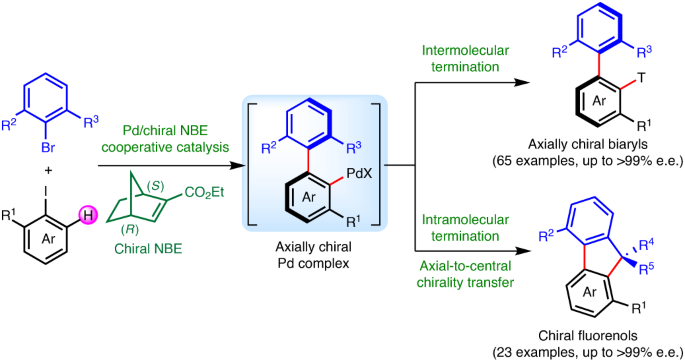
Construction of axial chirality via palladium/chiral norbornene cooperative catalysis | Nature Catalysis

China Tetrakis(triphenylphosphine)palladium(0) (Pd(PPh3)4) CAS No.: 14221-01-3 Manufacturers - Free Sample - Alfa Chemical
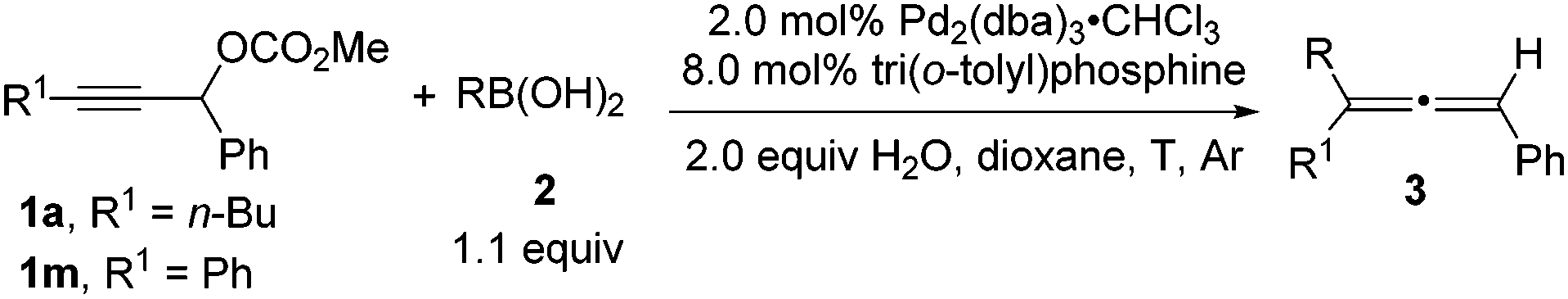
Tri(o-tolyl)phosphine for highly efficient Suzuki coupling of propargylic carbonates with boronic acids - Chemical Communications (RSC Publishing)
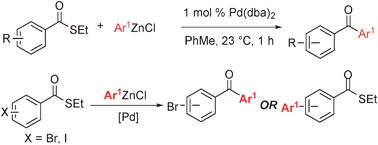
Synthesis of diaryl ketonesvia a phosphine-free Fukuyama reaction - Chemical Communications (RSC Publishing)

Carbonylation of terminal alkynes catalysed by Pd complexes in combination with tri(2-furyl)phosphine and methanesulfonic acid - ScienceDirect









![Phosphine Ligands [Cross-coupling Reaction using Transition Metal Catalysts] | TCI AMERICA Phosphine Ligands [Cross-coupling Reaction using Transition Metal Catalysts] | TCI AMERICA](https://www.tcichemicals.com/medias/T1643.jpg?context=bWFzdGVyfHJvb3R8Mzk2MTR8aW1hZ2UvanBlZ3xoZGMvaDA5Lzg5MzI0ODgyNDkzNzQvVDE2NDMuanBnfDFiZTAzOWNlZGFiNjZjZDMwYzZjZGIzNzY4MWVhMGVkZGEzNTRjMzg0YmVjNDYwNTNlYzk5MzEwOGIyN2EwMDM)
